- Atoms With The Same Number Of Protons But Different Number Neutrons
- Atoms With The Same Number Of Protons But Different Numbers Of Neutrons In The Nucleus Of An Atom
- How To Find Protons Neutrons And Electrons
- Atoms With The Same Number Of Protons But Different Numbers Of Neutrons
Learning Objectives
- Define atomic number.
- Define mass number.
- Determine the number of protons, neutrons, and electrons in an atom.
ISOTOPIC NOTATION isotopes are atoms with the same number of protons but different number of neutrons A Z X A = mass number Z = atomic number X = element symbol READING ISOTOPIC NOTATION 46 = (the total number of protons (21) + neutrons (25) 21 = (the total number of protons (21)) Sc = In a atom, the number. Atoms with the same number of protons but with different electrical charges Answer: are different ions. Learn More: Share this Share on Facebook Tweet on Twitter.
It's important to be able to distinguish atoms of one element from atoms of other elements. Elements are pure substances that make up all other matter, so each one is given a unique name. The names of elements are also represented by unique one- or two-letter symbols, such as (ce{H}) for hydrogen, (ce{C}) for carbon, or (ce{He}) for helium. However, it would more powerful if these names could be used to identify the numbers of protons and neutrons in the atoms. That's where atomic number and mass number come are useful.
Atomic Number

Scientists distinguish between different elements by counting the number of protons in the nucleus (Table (PageIndex{1})). If an atom has only one proton, we know it's a hydrogen atom. An atom with two protons is always a helium atom. If scientists count four protons in an atom, they know it's a beryllium atom. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom with six protons is a carbon atom . . . the list goes on.
Atoms With The Same Number Of Protons But Different Number Neutrons
Since an atom of one element can be distinguished from an atom of another element by the number of protons in its nucleus, scientists are always interested in this number, and how this number differs between different elements. The number of protons in an atom is called its atomic number ((Z)). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons.
| Name | Protons | Neutrons | Electrons | Atomic Number (Z) | Mass Number(A) |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | 1 |
| Helium | 2 | 2 | 2 | 2 | 4 |
| Lithium | 3 | 4 | 3 | 3 | 7 |
| Beryllium | 4 | 5 | 4 | 4 | 9 |
| Boron | 5 | 6 | 5 | 5 | 11 |
| Carbon | 6 | 6 | 6 | 6 | 12 |
Of course, since neutral atoms have to have one electron for every proton, an element's atomic number also tells you how many electrons are in a neutral atom of that element. For example, hydrogen has an atomic number of 1. This means that an atom of hydrogen has one proton, and, if it's neutral, one electron as well. Gold, on the other hand, has an atomic number of 79, which means that an atom of gold has 79 protons, and, if it's neutral, 79 electrons as well.
Neutral Atoms
Atoms are neutral in electrical charge because they have the same number of negative electrons as positive protons (Table (PageIndex{1})). Therefore, the atomic number of an atom also tells you how many electrons the atom has. This, in turn, determines many of the atom's chemical properties.
Mass Number
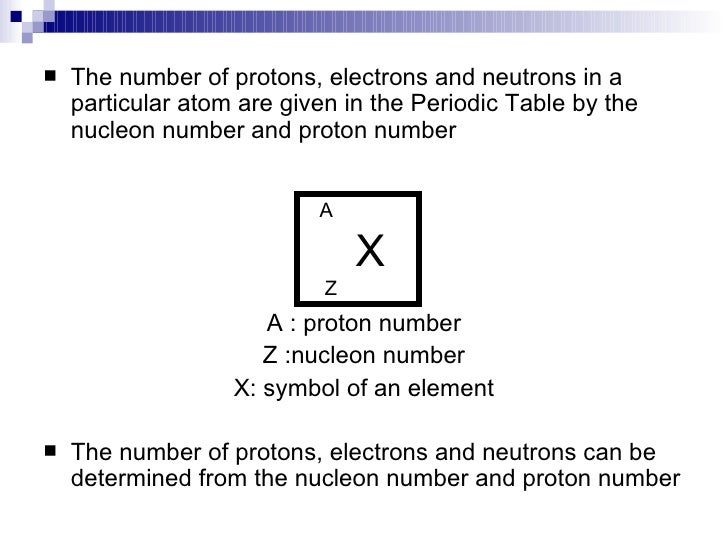
The mass number ((A)) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the atomic mass unit (left( text{amu} right)). One atomic mass unit is the mass of a proton, or about (1.67 times 10^{-27}) kilograms, which is an extremely small mass. A neutron has just a tiny bit more mass than a proton, but its mass is often assumed to be one atomic mass unit as well. Because electrons have virtually no mass, just about all the mass of an atom is in its protons and neutrons. Therefore, the total number of protons and neutrons in an atom determines its mass in atomic mass units (Table (PageIndex{1})).
Consider helium again. Most helium atoms have two neutrons in addition to two protons. Therefore the mass of most helium atoms is 4 atomic mass units ((2 : text{amu}) for the protons + (2 : text{amu}) for the neutrons). However, some helium atoms have more or less than two neutrons. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Because the number of neutrons can vary for a given element, the mass numbers of different atoms of an element may also vary. For example, some helium atoms have three neutrons instead of two (these are called isotopes and are discussed in detail later on)
Why do you think that the 'mass number' includes protons and neutrons, but not electrons? You know that most of the mass of an atom is concentrated in its nucleus. The mass of an atom depends on the number of protons and neutrons. You have already learned that the mass of an electron is very, very small compared to the mass of either a proton or a neutron (like the mass of a penny compared to the mass of a bowling ball). Counting the number of protons and neutrons tells scientists about the total mass of an atom.
[text{mass number} : A = left( text{number of protons} right) + left( text{number of neutrons} right)]
Atoms With The Same Number Of Protons But Different Numbers Of Neutrons In The Nucleus Of An Atom
An atom's mass number is very easy to calculate provided you know the number of protons and neutrons in an atom.
Example 4.5.1
What is the mass number of an atom of helium that contains 2 neutrons?
Solution
(left( text{number of protons} right) = 2) (Remember that an atom of helium always has 2 protons.)
(left( text{number of neutrons} right) = 2)
How To Find Protons Neutrons And Electrons
(text{mass number} = left( text{number of protons} right) + left( text{number of neutrons} right))
(text{mass number} = 2 + 2 = 4)
Summary
Atoms With The Same Number Of Protons But Different Numbers Of Neutrons
- Each element has a unique number of protons. An element's atomic number is equal to the number of protons in the nuclei of any of its atoms.
- The mass number of an atom is the sum of the protons and neutrons in the atom.
Contributors
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
